The most recent era of healthcare is personalized anesthesia, representing a movement from one-size-fits-all care to targeted, patient-centric therapies. Genomics is fundamental to this shift, defining what makes us unique from all others on Earth. Gene editing is among the most recently targeted therapies, an approach that may revolutionize healthcare. Let’s demystify the approach.
Table of Contents
- Innovative gene therapy
- The remarkable numerics of the human body
- A cautionary tale of the technology: Jesse Gelsinger
- Important questions to consider
- Gene therapy in personalized anesthesia—really?
- Where we are today
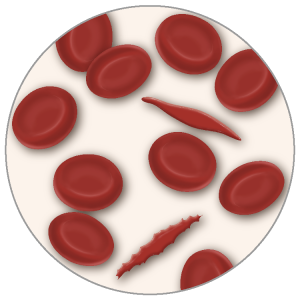
CRNAs who provide anesthesia care to those with sickle cell disease (SCD) know that there are life-threatening and debilitating effects of the condition. The sickling mutation causes red cells to assume a crescent (sickle) shape, leading to blood flow restriction and ischemic and vasooclusive events. Repeated, chronic ischemic events initially result in degraded quality of life, followed by often incapacitating disability and early mortality.
We worked for years in an institution with a preeminent SCD center and stayed abreast of therapies for this heritable disease. We were thrilled and intrigued when, in December 2023, the FDA approved the first two cell-based gene therapies, Casgevy and Lyfgenia, for the treatment of SCD.
Innovative gene therapy
Casgevy utilizes CRISPR/Cas9 technology, often referred to as “molecular scissors.” The discoverers of this technology were awarded the Nobel Prize in Chemistry in 2020. This process harvests a patient’s stem cells and modifies their DNA. The edited cells are then transplanted back into the patient, proliferating in the bone marrow and generating fetal hemoglobin (HbF), preventing sickling.
Lyfgenia takes a somewhat different approach. It uses a nonpathogenic virus to shuttle a genetic modification into the patient’s cells, where the gene therapy-derived hemoglobin is encoded and produced. This hemoglobin functions like adult hemoglobin (HbA) and is designated HbAT87Q.
Besides effecting a cure, a benefit of these innovations is that the process uses the patient’s stem cells, so immunological rejection is not a problem. A major drawback is that patients undergo initially debilitating chemotherapy to obliterate mutant cells from the bone marrow, which are then replaced by Casgevy- or Lyfgenia-modified cells. The treatments are also very expensive, with current estimates of Casgevy at $2.2 million and Lyfgenia at $3.1 million for each patient.
A wide range of side effects may occur, such as hair loss, infections, mouth ulcers, and, with Lyfgenia, possible blood cancers, necessitating its FDA Box Warning. Despite the side effects, these advances are considered significant medical breakthroughs with the potential to treat various conditions. Diabetes, rheumatoid arthritis, neurodegenerative conditions, and neuromuscular diseases come to mind. There are even forms of congenital blindness now believed to be amenable to gene therapy. We counted well over 100 diseases currently targeted with gene editing research and dozens of companies aggressive in their pursuit.
The remarkable numerics of the human body
Let’s digress for a moment and think about ourselves—not in terms of who we are or what we like or dislike, but rather the sheer numerical complexity of each of us. Contemplate our unique genome’s influence in maintaining health and generating disease. Consider our incredible complexity—and please excuse us if our math is not precise. We did double-check!
Humans:
- make red blood cells at an astounding, incomprehensible rate of about 2 million per second.
- are composed of 30 to 40 trillion cells, with RBCs being the largest single-cell type yet accounting for less than 5% of our total mass. The bacterial count within and on our bodies likely outnumbers our somatic cells.
- have smooth blood conduits throughout; if laid end-to-end, would circumnavigate the Earth over two times, about 60,000 miles.
- have abundant DNA that taxes our ability to fathom the size and length of this component. Each body cell contains about 2 meters of DNA. Assuming the lower estimate of 30 trillion cells, we have enough end-to-end DNA to go around the Earth 1.5 million times. Stated another way, it’s enough to go from the Earth to the Sun (93 million miles) almost 400 times. DNA is about 6 microns in diameter and is not an unmanageable tangle despite the tight quarters. It is packaged in a manner that permits ready access to the many enzymes in the cell that use it to do their work, namely encode proteins, do repairs, and even replicate.
A cautionary tale of the technology: Jesse Gelsinger
Jesse was one of the first patients to undergo gene editing to treat a rare metabolic disorder causing lethal ammonia levels to accumulate. Over half with the condition die within a year of birth. As a 17-year-old, Jesse’s condition was managed with a restrictive diet and four dozen pills each day.
Jesse enrolled in a trial at the University of Pennsylvania, where he underwent gene editing, and within a day, he experienced jaundice, intense systems-wide inflammation, and multiorgan failure; he died three days later. The news rocked the medical world, dealing a particularly severe blow to the gene editing community. Jennifer Doudna (a co-recipient of the Nobel Prize for CRISPR/Cas9), whose work was not involved, said, “Jesse’s death made the whole field of gene therapy go away, mostly, for at least a decade.”
Important questions to consider
Gene editing should prompt us to ask the tough questions. New healthcare interventions should be approached cautiously and conservatively, as the downstream consequences may not always be fully appreciated.
Relevant to gene editing:
- Could an inflammatory response be triggered by the virus used?
- Is there any chance the deactivated virus might reactivate, potentially causing disease?
- If a gene is inserted incorrectly, might it lead to cancer or some other unanticipated serious side effect?
- Should the process be utilized if it is too expensive, laborious, or insufficiently efficacious compared to traditional interventions?
- What are the ethics of manipulating DNA? If we can edit bad genes, can we not introduce designer genes to create ‘superhumans’? What about creating docile humans who mindlessly do what they are told to do?
- What might the outcomes be of the potential marriage of artificial intelligence with gene editing? Should we embrace this, or should we be wary.
The new generation of gene therapy is likely much safer than it was 25 years ago, with important and sobering lessons learned since Jesse’s 1999 death. But just how safe is it? Do the benefits sufficiently outweigh the risks in the era of personalized anesthesia? Do we even have the tools and knowledge to gauge the risk adequately?
Gene therapy in personalized anesthesia—really?
Advances in our understanding of genetics greatly impact the decisions that CRNAs make. This is the domain of pharmacogenomics and precision medicine. Knowledge of genetic variation in receptors and the activity of hepatic and blood enzymes reveals the reasons for many of the variable drug responses we see throughout the perioperative period.
Consider succinylcholine and how mutations in butyrylcholinesterase (pseudocholinesterase) encoders affect its activity. Ponder how mutations in the gene that metabolizes codeine necessitated an FDA Box Warning due to deaths in children. Then, reflect on the pharmacogenetic disorder, malignant hyperthermia (MH), and its patient safety implications. Appreciating the genetic fundamentals of a disorder is the start of a pathway to its genetic repair.
Research and clinical work on postop cancer recurrence are robust, and one investigator whose work we’ve followed at the University of California San Francisco envisions gene therapy playing a significant role that is, at this time, still largely theoretical. Other work worldwide involves treating acute and chronic pain, particularly neuropathic pain.
Given the opioid epidemic and the current limitations of good analgesic options for chronic pain, newer experimental agents will continue to emerge. By targeting a specific receptor or another molecular target, gene therapy may provide excellent analgesic efficacy for pain treatment without the side effects seen with current pharmacotherapies. This may be a particularly fertile domain for clinical application.
Consider just one application: phantom limb pain. Imagine a patient scheduled for limb amputation who, in the days or weeks before the procedure, undergoes gene editing to produce long-term postoperative analgesia. On the day of surgery, you employ a regional or general technique and a multimodal approach for perioperative analgesia. The patient is comfortable with short and long-term follow-up, revealing no need for opioids and no symptoms associated with the phantom limb syndrome. Is this even possible? Yes, this is likely to happen.
Here is a short list of potential targets of gene therapy in anesthesiology: increasing myocardial tolerance to ischemia, preventing acute kidney injury, extinguishing a drug allergy, eliminating nausea and vomiting, and transforming a patient at risk for MH into one who is not. Science fiction? Not from what we see happening in the growing literature on genome editing.
Where we are today
The clinical applications of genetically editing specific molecular and biochemical targets are slowly but inexorably moving to the patient’s bedside. Given the ample clinical need, chronic pain represents a domain sorely in need of additional therapies and may be the first syndrome to emerge with FDA-approved tools for anesthesiology.
Even if the merits of direct clinical application of gene editing seem remote, consider this: basic science research has a long and robust history of creating pathways and opening doors to applications previously not even considered. Stay tuned; you may be surprised at what lies beyond the horizon, but what may soon come into view.