There is an undercurrent of neuroscience-grounded activity underway in our professional community. Niches of CRNAs nationwide, working alone or in concert with like-minded physicians, are challenging some of the historical and contemporary foundations of general anesthesia. Largely quiet in their activities, representing a very small circle inside a much larger circle on a Venn diagram, they are pursuing their ambition to eliminate the use of potent inhaled agents in their practices and turning to total intravenous anesthesia (TIVA).
Table of Contents
- What we learn about general anesthesia in our basic programs
- Targeting specific receptors and an introduction to our term, “C-FAN”
- The downsides to potent inhaled agents
- The emergence of clinician-friendly measurements of CNS activity
- C-FAN is not a processed EEG!
- Should our ether hybrids be abandoned?
While rubrics like “radical,” “maverick,” or “intrepid” come to mind, these fall short of singularly capturing the mindset, passion, intellect, and purpose of these devotees. For now, we will dispense with labeling them; instead, leave that up to you as we describe just what these clinical practitioners and thinkers are up to.
What we learn about general anesthesia in our basic programs
Let’s journey back to your early training days when you first truly appreciated what general anesthesia was all about. Regardless of your vintage, you likely learned a contemporary descendant of an ether-based compound brought it on. We’ll give a quick shout-out to halothane (an alkane), but otherwise, we’ll confine our discussion to the three halogenated ethers in current use—isoflurane, sevoflurane, and desflurane.
You came to know that ether-based anesthetics are “total” anesthetics. In a dose-dependent manner, they produce unconsciousness, amnesia, analgesia, areflexia, and akinesia, collectively representing the required components for a general anesthetic state. Miss one of these targets, and oops, something important is missing. But in the case of our current ether hybrids, one drug does it all.
These clinical goals are not achieved with a uniform dose; each pharmacological target demands a specific concentration at the respective effect site. The dose of sevoflurane needed to achieve akinesia differs from that required to produce unconsciousness, and so forth. One MAC of an agent fails to address the individual components of general anesthesia and only gives us an overall potency metric in preventing purposeful movement to a nociceptive stimulus like a skin incision. Achieving a particular clinical target with a potent inhaled agent may result in unacceptable hypotension.
Targeting specific receptors and an introduction to our term, “C-FAN”
We circumvent this limitation by using what some refer to as “balanced” anesthesia (old-timers may prefer “garbage anesthesia”), in which we add specific drugs targeting unique receptors that mediate the precise response we want. Nicotinic, muscarinic, GABA, NMDA, 5-HT3, and alpha-2 receptors, among others, come to mind.
We have two broad approaches to achieving a general anesthetic state: inhalational and TIVA. Both are safe, titratable, and effective. These approaches have been with us for a long while, with the inhalational approach having about a century’s head start! But what’s happened over the last 25 years is a systematic and methodologically sophisticated understanding of what’s going on grounded in what we term “Clinician-Friendly Actionable Neuroscience,” or, for simplicity, C-FAN.
Googling C-FAN, you’re not going to come up with anything as it’s a term we created, and currently, it’s more of an evolving idea than a thing. We’ve only now put it out there for others—like you—to consider. So, what’s going on here, and what’s up with C-FAN?
The downsides to potent inhaled agents
First, there are a lot of concerns about traditional, potent inhaled agents. Among these are that in animal models and even in some human observational studies, there are concerns that general anesthesia induced by our potent inhalants may have neurodegenerative effects. Some suggest that these effects may be particularly concerning in young children (subsequent learning impairment, attention deficit disorder, behavioral issues) and older adults (cognitive impairment), prompting an FDA warning that’s just a hair shy of a boxed warning. The literature also suggests a high incidence of emergence delirium—acutely or in the first hours or day or so)—in pediatric and adult patients.
There are also concerns that long-term occupational exposure to even trace amounts of these agents may have biological consequences for us and other members of the operative team. Added to this is the now well-appreciated contribution that the potent inhaled agents have on greenhouse gases and their deleterious effects on the ozone level when they are vented to the outside air ending up in our atmosphere. Some even suggest they play a consequential role in what is described as global warming. Then there is the issue of cardiovascular depression, often caused by incidental overdosing to achieve a desired state with attendant adverse events like acute kidney injury.
These issues are widely discussed in the literature and have gained traction in the minds of some providers who, armed with C-FAN, have sought alternatives. These forward-thinking, early adopters of TIVA, to the point of eliminating potent inhaled agents from their practice, come grounded in what they believe is an evidence-based, practical, safe, and highly effective respite from inhalants. They’ve become iconoclasts with total avoidance of the potent agents from their practices. Their view is to use the tools and understanding that neuroscience offers to employ precision medicine in their clinical care of patients.
The emergence of clinician-friendly measurements of CNS activity
There is a bit of an irony that everything we’ve ever seen and know about life on planet Earth comes to us from an organ that resides in an encapsulated enclosure devoid of light. Its matrix is such that if you were to scoop it out of its enclave and hold it in your hands (use both, or you might drop it!), you’d perceive it as feeling a bit—on the “tofu scale”—as middling between regular and firm. But continually coursing through this extraordinary organ, via its neuronal circuitry, is an inconceivably large flood of electrical activity. That activity can be captured by now common and relatively compact devices that display the electrical activity permitting clinician analysis, and providing a real-time opportunity to achieve targeted outcomes pharmacologically.
The entire May 15, 2024 issue of Neuron, a high-impact journal, is devoted to clarifying what consciousness is and how unconsciousness is induced. Using easily acquired electrical signaling, MIT researchers revealed that disrupting the brain’s normal balance between stability and excitability makes its activity increasingly unstable until unconsciousness ensues. The researchers note that the brain operates on what might be termed a “knife’s edge” between excitability and chaos. It must be excitable for neurons to interact with each other, but if it becomes too excitable, chaos can rule.
This research underscores the value of using and analyzing specific electrical patterns induced by drugs such as propofol or those targeting the other receptors mentioned earlier. It offers a pathway to developing ways to control a patient’s level of anesthesia more precisely.
The foundations for these TIVA devotees come from a large body of bench and clinical research grounded in neuroscience, a field of biology that is only now beginning to receive its due recognition in our domain. What we now know is that:
- When humans “go under” general anesthesia, the brain is hardly “turned off” but rather pulses with dynamic electrical activity
- The drugs we use alter our normal arousal by inducing and maintaining oscillations in electrical activity.
- The activity is easily captured and observed with electroencephalography (EEG) acting like a simple voltmeter.
- The oscillations have “signatures” that permit tracking of the anesthetic state.
- A drug like propofol, or sevoflurane, acting on the GABA-A receptors allows negatively charged chloride ions to enter the cells of the CNS and quiet them–observable with that voltmeter.
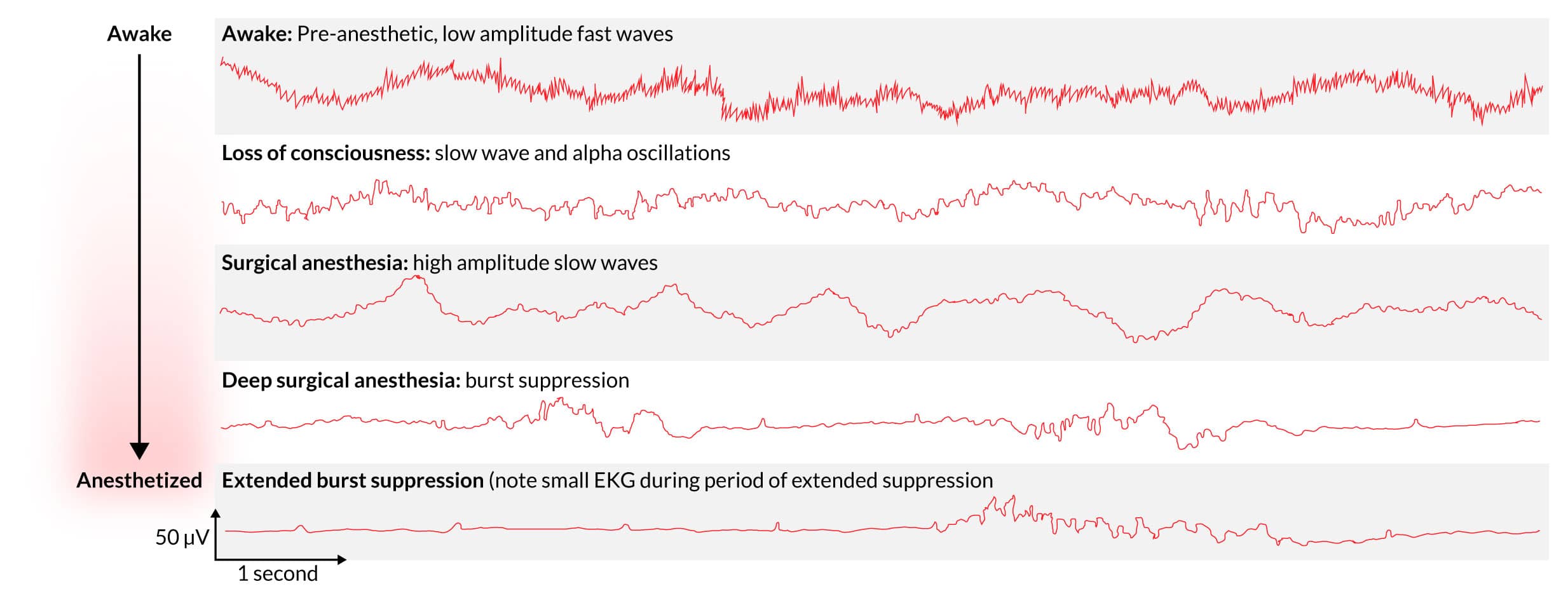
- The oscillations change as the anesthetic depresses the CNS, with characteristic waveforms occurring.
- The waves initially get bigger, with slow oscillations revealing a depressed brain stem. Finally, burst suppression reveals a profound state of brain inactivation.
What is happening is some of these early adopters propose a new and enlightened, neuroscience-based approach to brain monitoring of patients receiving general anesthesia or sedation. They propose to train CRNAs to recognize and analyze anesthetic-induced brain states with drug-specific neurophysiological signatures gathered with an unprocessed 4-channel EEG and a spectrogram. Achieving and monitoring a desired anesthetic state using these unique signatures mirrors the molecular and neural circuit mechanisms of the activity of our drugs.
The ability to make detailed and accurate determinations is advocated by these aficionados as a way to optimize our ability to hit targets precisely and design patient-centric anesthetic care using a multimodal approach. They emphasize precise control of antinociception and simultaneously advantage the precise pharmacokinetics as the patient is controllably aroused from anesthesia.
In 2024 many envision raw EEG monitoring becoming commonplace in monitoring drug effects and see multimodal TIVA as a neurophysiological way to combine, control, and optimize the delivery of the agents. This permits second-to-second targeting of anesthetic depth and exacting control over the speed of emergence, with the hope of decreasing the adverse effects of the potent vapors.
C-FAN is not a processed EEG!
There are now compact, battery-powered EEG monitors, wireless with comfortable and adjustable headsets that leave no residue. This is not a BIS monitor or other proprietary-based, processed EEG device prone to measurement and interpretive pitfalls. C-FAN uses a raw EEG that is easy to use and interpretable with minimal training for the CRNA to gain the facility for its interpretation sufficient to guide precision TIVA. Proponents argue this technology is like moving away from auscultating BPs to automated noninvasive BP determinations, etCO2 replacing arterial blood gas analysis, and pulse oximetry supplanting indirect clinical estimations of arterial oxygen content. CRNAs use highly sophisticated technologies with aplomb—think ultrasound guidance for nerve blocks, line placements, and many other assessments. Our technological skill sets also include cardiac ECHO, TEE, quantitative neuromuscular function monitoring, and advanced ECG interpretation. Simplified EEG analysis is likewise on the horizon, and sufficient mastery is highly achievable.
C-FAN is neuroscience marshaled to the patient’s bedside, precisely using a cocktail of agents, including IV propofol, short-acting analgesics, drugs that create akinesia, and others that target specific regions of the brain, like ketamine and dexmedetomidine. This provides an alternative to inhaled agents using raw 4-channel EEG signals captured in real-time with C-FAN-friendly tools. The site of action of our anesthetics is the CNS; we should and can specifically target the effect site using emerging technologies. And once AI gets pulled into the picture, its use will be even more actionable.
Should our ether hybrids be abandoned?
No!! We are not advocating for eliminating these time-tested, reliable, and titratable members of our pharmacopeia, nor are we suggesting that TIVA is optimal for all cases and practices. We are reporting on what appears to be an undercurrent of clinical practice that is growing domestically and internationally. C-FAN monitoring tools are coming our way just as 1973 heralded the first use of the cell phone and the avalanche of technology that it foretold.
Does this represent an existential threat to “traditional” general anesthesia induction and maintenance? Or is it just the ever-evolving nature of what we do? It may be coming to an OR close to you if not already there.
As CRNAs ourselves, we understand the challenge of fitting CRNA continuing education credits into your busy schedule. When you’re ready, we’re here to help.